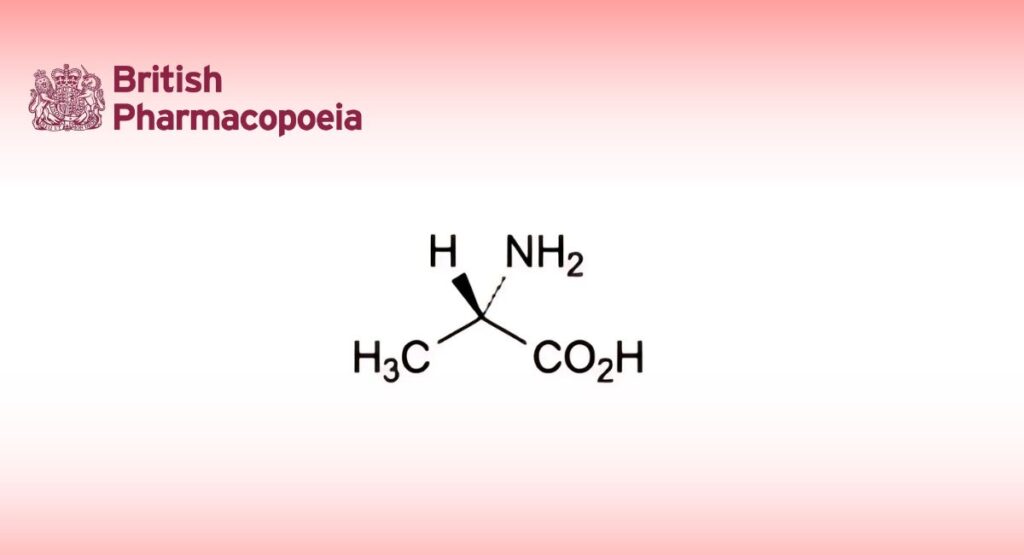
(Ph. Eur. monograph 0752)
C3H7NO2 89.1 56-41-7
Action and use
Amino acid.
DEFINITION
(2S)-2-Aminopropanoic acid.
Content
98.5 per cent to 101.0 per cent (dried substance).
CHARACTERS
Appearance
White or almost white, crystalline powder or colourless crystals.
Solubility
Freely soluble in water, very slightly soluble in ethanol (96 per cent).
IDENTIFICATION
First identification: A, B.
Second identification: A, C, D.
A. Specific optical rotation (see Tests).
B. Infrared absorption spectrophotometry (2.2.24).
Comparison alanine CRS.
C. Thin-layer chromatography (2.2.27).
Test solution: Dissolve 10 mg of the substance to be examined in water R and dilute to 50 mL with the same solvent.
Reference solution: Dissolve 10 mg of alanine CRS in water R and dilute to 50 mL with the same solvent.
Plate: TLC silica gel plate R.
Mobile phase: glacial acetic acid R, water R, butanol R (20:20:60 V/V/V).
Application: 5 μL.
Development: Over 2/3 of the plate.
Drying: In air.
Detection: Spray with ninhydrin solution R and heat at 105 °C for 15 min.
Results: The principal spot in the chromatogram obtained with the test solution is similar in position, colour and size to the principal spot in the chromatogram obtained with the reference solution.
D. Dissolve 0.5 g in a mixture of 0.25 mL of hydrochloric acid R1, 0.5 mL of a 100 g/L solution of sodium nitrite R and 1 mL of water R. Shake; gas is given off. Add 2 mL of dilute sodium hydroxide solution R, followed by 0.25 mL of iodinated potassium iodide solution R. After about 30 min, a yellow precipitate is formed.
TESTS
Solution S
Dissolve 2.5 g in distilled water R and dilute to 50 mL with the same solvent.
Appearance of solution
The solution is clear (2.2.1) and not more intensely coloured than reference solution BY6 (2.2.2, Method II)
Dilute 10 mL of solution S to 20 mL with water R.
Specific optical rotation (2.2.7)
+ 13.5 to + 15.5 (dried substance).
Dissolve 2.50 g in hydrochloric acid R1 and dilute to 25.0 mL with the same acid.
Ninhydrin-positive substances
Amino acid analysis (2.2.56). For analysis, use Method 1.
The concentrations of the test solution and the reference solutions may be adapted according to the sensitivity of the equipment used. The concentrations of all solutions are adjusted so that the system suitability requirements described in general chapter 2.2.46 are fulfilled, keeping the ratios of concentrations between all solutions as described.
Solution A: dilute hydrochloric acid R1 or a sample preparation buffer suitable for the apparatus used. Test solution Dissolve 30.0 mg of the substance to be examined in solution A and dilute to 50.0 mL with solution A. Reference solution (a) Dilute 1.0 mL of the test solution to 100.0 mL with solution A. Dilute 2.0 mL of this solution to 10.0 mL with solution A.
Reference solution (b): Dissolve 30.0 mg of proline R in solution A and dilute to 100.0 mL with solution A. Dilute 1.0 mL of the solution to 250.0 mL with solution A.
Reference solution (c): Dilute 6.0 mL of ammonium standard solution (100 ppm NH4) R to 50.0 mL with solution A. Dilute 1.0 mL of this solution to 100.0 mL with solution A.
Reference solution (d): Dissolve 30 mg of isoleucine R and 30 mg of leucine R in solution A and dilute to 50.0 mL with solution A. Dilute 1.0 mL of the solution to 200.0 mL with solution A.
Blank solution: Solution A.
Inject suitable, equal amounts of the test, blank and reference solutions into the amino acid analyser. Run a program suitable for the determination of physiological amino acids.
System suitability Reference solution (d):
— resolution: minimum 1.5 between the peaks due to isoleucine and leucine.
Calculation of percentage contents:
— for any ninhydrin-positive substance detected at 570 nm, use the concentration of alanine in reference solution (a);
— for any ninhydrin-positive substance detected at 440 nm, use the concentration of proline in reference solution (b);
if a peak is above the reporting threshold at both wavelengths, use the result obtained at 570 nm for quantification.
Limits:
— any ninhydrin-positive substance: for each impurity, maximum 0.10 per cent;
— total: maximum 0.5 per cent;
— reporting threshold: 0.05 per cent.
Chlorides (2.4.4)
Maximum 200 ppm.
Dilute 5 mL of solution S to 15 mL with water R.
Sulfates (2.4.13)
Maximum 300 ppm.
Dilute 10 mL of solution S to 15 mL with distilled water R.
Ammonium
Amino acid analysis (2.2.56) as described in the test for ninhydrin-positive substances with the following modifications.
Injection Test solution, reference solution (c) and blank solution.
Limit:
— ammonium at 570 nm: not more than the area of the corresponding peak in the chromatogram obtained with
reference solution (c) (0.02 per cent), taking into account the peak due to ammonium in the chromatogram obtained with the blank solution.
Iron (2.4.9)
Maximum 10 ppm.
In a separating funnel, dissolve 1.0 g in 10 mL of dilute hydrochloric acid R. Shake with 3 quantities, each of 10 mL, of methyl isobutyl ketone R1, shaking for 3 min each time. To the combined organic layers add 10 mL of water R and shake for 3 min. Use the aqueous layer.
Loss on drying (2.2.32)
Maximum 0.5 per cent, determined on 1.000 g by drying in an oven at 105 °C.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Dissolve 80.0 mg in 3 mL of anhydrous formic acid R. Add 30 mL of anhydrous acetic acid R. Titrate with 0.1 M perchloric acid, determining the end-point potentiometrically (2.2.20).
1 mL of 0.1 M perchloric acid is equivalent to 8.91 mg of C3H7NO2.
STORAGE
Protected from light.
IMPURITIES
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities and/or by the general monograph Substances for pharmaceutical use (2034). It is therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10. Control of impurities in substances for pharmaceutical use) A, B.
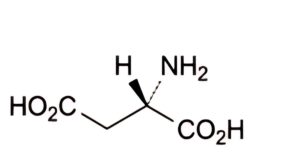
A. (2S)-2-aminobutanedioic acid (aspartic acid),
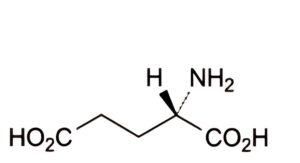
B. (2S)-2-aminopentanedioic acid (glutamic acid).