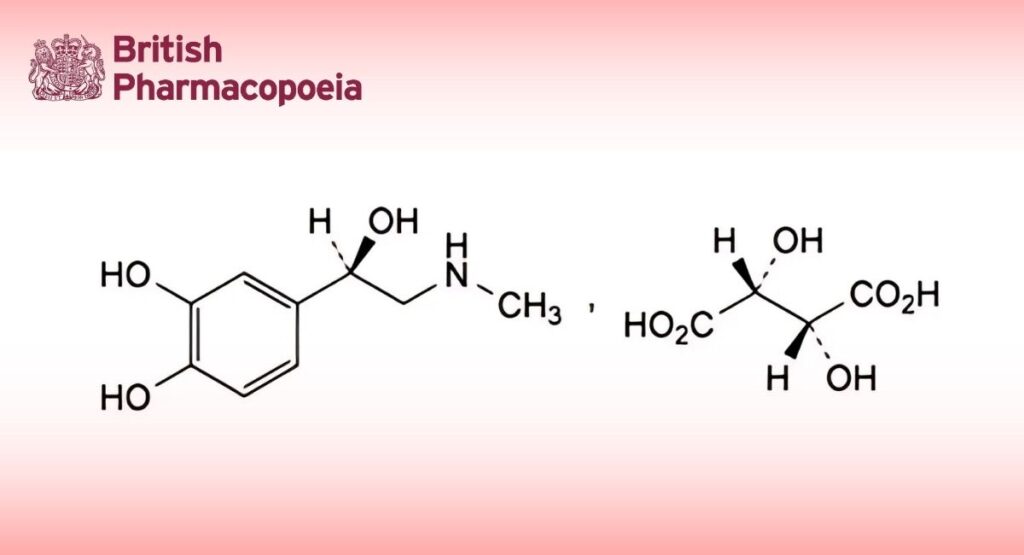
(Adrenaline Tartrate, Ph. Eur. monograph 0254)
C13H19NO9 333.3 51-42-3
Action and use
Adrenoceptor agonist.
Preparations
Adrenaline Injection/Epinephrine Injection
Dilute Adrenaline Injection (1 in 10,000)/Dilute Epinephrine Injection (1 in 10,000)
Adrenaline Solution/Epinephrine Solution
Adrenaline and Cocaine Intranasal Solution
Bupivacaine and Adrenaline Injection/Bupivacaine and Epinephrine Injection
Lidocaine and Adrenaline Injection/Lidocaine and Epinephrine Injection
DEFINITION
(1R)-1-(3,4-Dihydroxyphenyl)-2-(methylamino)ethanol hydrogen (2R,3R)-2,3-dihydroxybutanedioate.
Content
98.5 per cent to 101.0 per cent (dried substance).
CHARACTERS
Appearance
White or greyish-white, crystalline powder.
Solubility
Freely soluble in water, slightly soluble in ethanol (96 per cent).
IDENTIFICATION
A. Dissolve 5 g in 50 mL of a 5 g/L solution of sodium metabisulfite R and make alkaline by addition of ammonia R. Keep the mixture at room temperature for at least 15 min and filter. Reserve the filtrate for identification test C. Wash the precipitate with 3 quantities, each of 10 mL, of methanol R. Dry at 80 °C. The specific optical rotation (2.2.7) of the residue (adrenaline base) is -53.5 to -50, determined using a 20.0 g/L solution in 0.5 M hydrochloric acid.
B. Infrared absorption spectrophotometry (2.2.24).
Preparation: Discs of adrenaline base prepared as described under identification test A.
Comparison: Use adrenaline base prepared as described under identification test A from 50 mg of adrenaline
tartrate CRS dissolved in 5 mL of a 5 g/L solution of sodium metabisulfite R. Keep the mixture at room temperature for at least 30 min. Filter through a sintered-glass filter (2.1.2).
C. 0.2 mL of the filtrate obtained in identification test A gives reaction (b) of tartrates (2.3.1).
TESTS
Appearance of solution
The solution is not more opalescent than reference suspension II (2.2.1) and not more intensely coloured than reference solution BY5 (2.2.2, Method II).
Dissolve 0.5 g in water R and dilute to 10 mL with the same solvent. Examine the solution immediately.
Related substances
Liquid chromatography (2.2.29). Prepare the solutions protected from light.
Solvent mixture A: Dissolve 5.0 g of potassium dihydrogen phosphate R and then 2.6 g of sodium octanesulfonate R in water for chromatography R, and dilute to 1000 mL with the same solvent (it is usually necessary to stir for at least 30 min to achieve complete dissolution). Adjust to pH 2.8 with phosphoric acid R.
Solvent mixture B: acetonitrile R1, solvent mixture A (130:870 V/V).
Test solution: Dissolve 75 mg of the substance to be examined in 5 mL of 0.1 M hydrochloric acid and dilute to 50 mL with solvent mixture B.
Reference solution (a): Dilute 1.0 mL of the test solution to 100.0 mL with solvent mixture B. Dilute 1.0 mL of this solution to 10.0 mL with solvent mixture B.
Reference solution (b): Dissolve 1.5 mg of noradrenaline tartrate CRS (impurity B) and 1.5 mg of adrenalone
hydrochloride R (impurity C) in solvent mixture B, add 1.0 mL of the test solution and dilute to 100.0 mL with solvent mixture B.
Reference solution (c): Dissolve the contents of a vial of adrenaline impurity mixture CRS (impurities D and E) in 0.1 mL of 0.1 M hydrochloric acid and 0.9 mL of solvent mixture B.
Reference solution (d): Dissolve 7.5 mg of adrenaline tartrate with impurity A CRS in 0.5 mL of 0.1 M hydrochloric acid and dilute to 5.0 mL with solvent mixture B.
Blank solution: 0.1 M hydrochloric acid, solvent mixture B (1:9 V/V).
Column:
— size: l = 0.10 m, Ø = 4.6 mm;
— stationary phase: end-capped octadecylsilyl silica gel for chromatography R (3 μm);
— temperature: 50 °C.
Mobile phase:
— mobile phase A: acetonitrile R1, solvent mixture A (5:95 V/V);
— mobile phase B: acetonitrile R1, solvent mixture A (45:55 V/V);
| Time
(min) |
Mobile phase A
(per cent V/V) |
Mobile phase B
(per cent V/V) |
| 0 – 15
15 – 20 20 – 25 |
92 → 50
50 → 92 92 |
8 → 50
50 → 8 8 |
Flow rate: 2.0 mL/min.
Detection: Spectrophotometer at 210 nm.
Injection: 20 μL.
Identification of impurities: Use the chromatogram supplied with adrenaline impurity mixture CRS and the chromatogram obtained with reference solution (c) to identify the peaks due to impurities D and E; use the chromatogram supplied with adrenaline tartrate with impurity A CRS and the chromatogram obtained with reference solution (d) to identify the peak due to impurity A.
Relative retention: With reference to adrenaline (retention time = about 4 min): impurity B = about 0.8;
impurity C = about 1.3; impurity A = about 3.2; impurity D = about 3.3; impurity E = about 3.7.
System suitability: Reference solution (b):
— resolution: minimum 3.0 between the peaks due to impurity B and adrenaline.
Limits:
— correction factors: for the calculation of content, multiply the peak areas of the following impurities by the
corresponding correction factor: impurity D = 0.7; impurity E = 0.6;
— impurity A: not more than 3 times the area of the principal peak in the chromatogram obtained with reference
solution (a) (0.3 per cent);
— impurities B, C: for each impurity, not more than twice the area of the principal peak in the chromatogram obtained with reference solution (a) (0.2 per cent);
— impurities D, E: for each impurity, not more than the area of the principal peak in the chromatogram obtained with reference solution (a) (0.1 per cent);
— unspecified impurities: for each impurity, not more than the area of the principal peak in the chromatogram obtained with reference solution (a) (0.10 per cent);
— total: not more than 6 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.6 per cent);
— disregard limit: 0.5 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.05 per cent).
Loss on drying (2.2.32)
Maximum 0.5 per cent, determined on 1.000 g by drying in vacuo for 18 h.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Dissolve 0.300 g in 50 mL of anhydrous acetic acid R, heating gently if necessary. Titrate with 0.1 M perchloric acid until a bluish-green colour is obtained, using 0.1 mL of crystal violet solution R as indicator.
1 mL of 0.1 M perchloric acid is equivalent to 33.33 mg of C13H19NO9.
STORAGE
In an airtight container, or preferably in a sealed tube under vacuum or under an inert gas, protected from light.
IMPURITIES
Specified impurities A, B, C, D, E.
A. unknown structure,
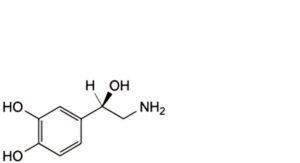
B. (1R)-2-amino-1-(3,4-dihydroxyphenyl)ethanol (noradrenaline),
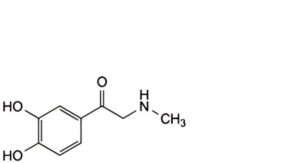
C. 1-(3,4-dihydroxyphenyl)-2-(methylamino)ethanone (adrenalone),
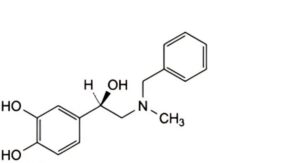
D. 4-[(1R)-2-(benzylmethylamino)-1-hydroxyethyl]benzene-1,2-diol,
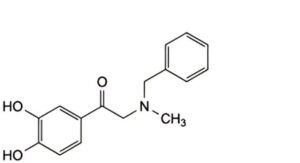
E. 2-(benzylmethylamino)-1-(3,4-dihydroxyphenyl)ethanone.