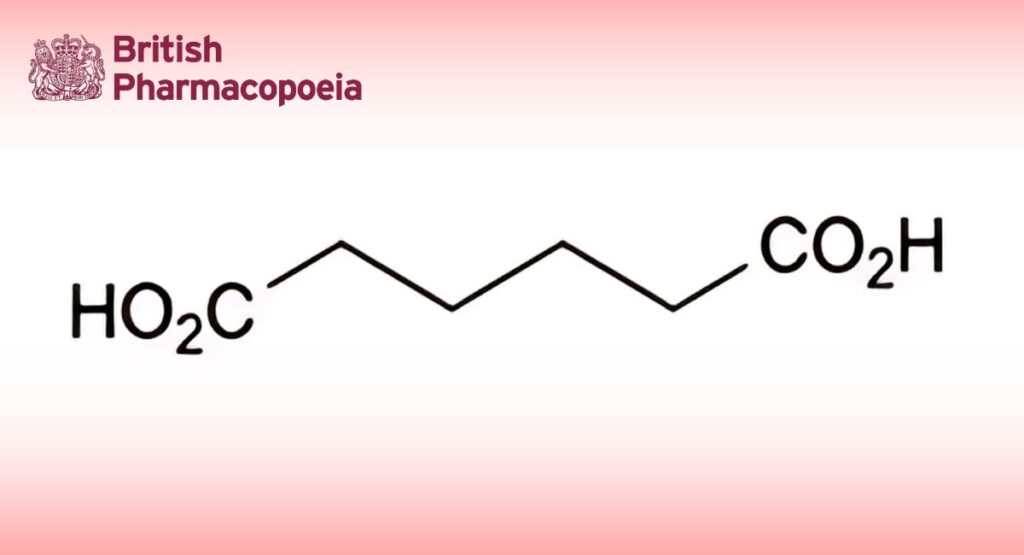
(Ph. Eur. monograph 1586)
C6H10O4 146.1 124-04-9
Action and use
Excipient.
DEFINITION
Hexanedioic acid.
Content
99.0 per cent to 101.0 per cent (dried substance).
CHARACTERS
Appearance
White or almost white, crystalline powder.
Solubility
Sparingly soluble in water, soluble in boiling water, freely soluble in ethanol (96 per cent) and in methanol, soluble in acetone.
IDENTIFICATION
A. Melting point (2.2.14): 151 °C to 154 °C.
B. Infrared absorption spectrophotometry (2.2.24).
Comparison adipic acid CRS.
TESTS
Solution S
Dissolve 5.0 g with heating in distilled water R and dilute to 50 mL with the same solvent. Allow to cool and to crystallise. Filter through a sintered-glass filter (40) (2.1.2). Wash the filter with distilled water R. Collect the filtrate and the washings until a volume of 50 mL is obtained.
Appearance of solution
The solution is clear (2.2.1) and colourless (2.2.2, Method II).
Dissolve 1.0 g in methanol R and dilute to 20 mL with the same solvent.
Related substances
Liquid chromatography (2.2.29).
Test solution Dissolve 0.20 g of the substance to be examined in the mobile phase and dilute to 10.0 mL with the mobile phase.
Reference solution (a) Dissolve 20 mg of glutaric acid R in 1.0 mL of the test solution and dilute to 10.0 mL with the mobile phase.
Reference solution (b) Dilute 1.0 mL of the test solution to 100.0 mL with the mobile phase, dilute 1.0 mL of the solution to 10.0 mL with the mobile phase.
Column:
— size: l = 0.125 m, Ø = 4.0 mm,
— stationary phase: spherical octadecylsilyl silica gel for chromatography R (5 μm) with a specific surface area of 350 m /g and a pore size of 10 nm,
— temperature: 30 °C.
Mobile phase Mix 3 volumes of acetonitrile R and 97 volumes of a 24.5 g/L solution of dilute phosphoric acid R.
Flow rate 1 mL/min.
Detection Spectrophotometer at 209 nm.
Injection 20 μL.
Run time 3 times the retention time of adipic acid.
System suitability Reference solution (a):
— resolution: minimum 9.0 between the peaks due to glutaric acid and adipic acid.
Limits:
— any impurity: not more than the area of the principal peak in the chromatogram obtained with reference solution (b) (0.1 per cent),
— total: not more than 5 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.5 per cent),
— disregard limit: 0.5 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.05 per cent).
Chlorides (2.4.4)
Maximum 200 ppm.
Dilute 2.5 mL of solution S to 15 mL with water R.
Nitrates
Maximum 30 ppm.
To 1 mL of solution S add 2 mL of concentrated ammonia R, 0.5 mL of a 10 g/L solution of manganese sulfate R, 1 mL of a 10 g/L solution of sulfanilamide R and dilute to 20 mL with water R. Add 0.10 g of zinc powder R and cool in iced water for 30 min; shake from time to time. Filter and cool 10 mL of the filtrate in iced water. Add 2.5 mL of hydrochloric acid R1 and 1 mL of a 10 g/L solution of naphthylethylenediamine dihydrochloride R. Allow to stand at room temperature. After 15 min the mixture is not more intensely coloured than a standard prepared at the same time and in the same manner, using 1.5 mL of nitrate standard solution (2 ppm NO3) R instead of 1 mL of solution S. The test is invalid if a blank solution prepared at the same time and in the same manner, using 1 mL of water R instead of 1 mL of solution S, is more intensely coloured than a 2 mg/L solution of potassium permanganate R.
Sulfates (2.4.13)
Maximum 500 ppm.
Dilute 3 mL of solution S to 15 mL with distilled water R.
Iron (2.4.9)
Maximum 10 ppm, determined on solution S.
Loss on drying (2.2.32)
Maximum 0.2 per cent, determined on 1.000 g by drying in an oven at 105 °C.
Sulfated ash (2.4.14)
Maximum 0.1 per cent.
Melt 1.0 g completely over a gas burner, then ignite the melted substance with the burner. After ignition, lower or remove the flame in order to prevent the substance from boiling and keep it burning until completely carbonised. Carry out the test for sulfated ash using the residue.
ASSAY
Dissolve 60.0 mg in 50 mL of water R. Add 0.2 mL of phenolphthalein solution R and titrate with 0.1 M sodium hydroxide. 1 mL of 0.1 M sodium hydroxide is equivalent to 7.31 mg of C6H10O4.
IMPURITIES
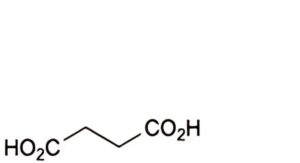
A. pentanedioic acid (glutaric acid),
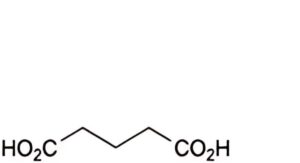
B. butanedioic acid (succinic acid),
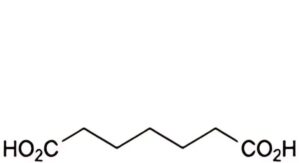
C. heptanedioic acid (pimelic acid).