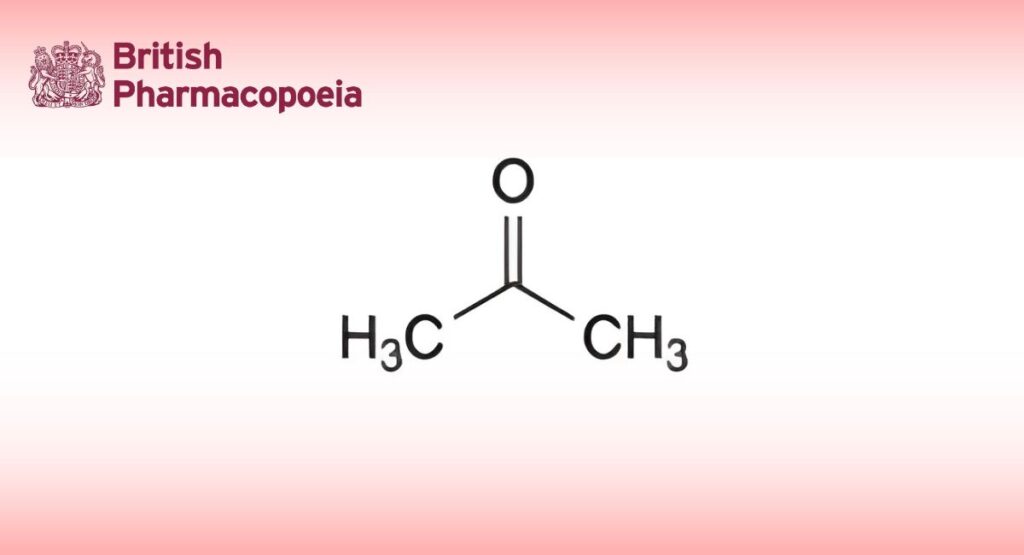
(Ph. Eur. monograph 0872)
C3H6O 58.08 67-64-1
DEFINITION
Propanone.
CHARACTERS
Appearance
Volatile, clear, colourless liquid.
Solubility
Miscible with water and with ethanol (96 per cent).
The vapour is flammable.
IDENTIFICATION
A. Relative density (see Tests).
B. To 1 mL, add 3 mL of dilute sodium hydroxide solution R and 0.3 mL of a 25 g/L solution of sodium nitroprusside R. An intense red colour is produced which becomes violet with the addition of 3.5 mL of acetic acid R.
C. To 10 mL of a 0.1 per cent V/V solution of the substance to be examined in ethanol (50 per cent V/V) R, add 1 mL of a 10 g/L solution of nitrobenzaldehyde R in ethanol (50 per cent V/V) R and 0.5 mL of strong sodium hydroxide solution R. Allow to stand for about 2 min and acidify with acetic acid R. A greenish-blue colour is produced.
TESTS
Appearance of solution
To 10 mL add 10 mL of water R. The solution is clear (2.2.1) and colourless (2.2.2, Method II).
Acidity or alkalinity
To 5 mL add 5 mL of carbon dioxide-free water R, 0.15 mL of phenolphthalein solution R and 0.5 mL of 0.01 M sodium hydroxide. The solution is pink. Add 0.7 mL of 0.01 M hydrochloric acid and 0.05 mL of methyl red solution R. The solution is red or orange.
Relative density (2.2.5)
0.790 to 0.793.
Reducing substances
To 30 mL add 0.1 mL of 0.02 M potassium permanganate and allow to stand in the dark for 2 h. The mixture is not completely decolourised.
Related substances
Gas chromatography (2.2.28).
Test solution: The substance to be examined.
Reference solution (a): To 0.5 mL of methanol R add 0.5 mL of 2-propanol R and dilute to 100.0 mL with the test solution.Dilute 1.0 mL of this solution to 10.0 mL with the test solution.
Reference solution (b): Dilute 100 μL of benzene R to 100.0 mL with the test solution. Dilute 0.20 mL of this solution to 100.0 mL with the test solution.
Column:
— material: fused silica,
— size: l = 50 m, Ø = 0.3 mm,
— stationary phase: macrogol 20 000 R (film thickness 1 μm).
Carrier gas helium for chromatography R.
Linear velocity: 21 cm/s.
Split ratio: 1:50.
Temperature:
| Time (min) |
Temperature (°C) |
|
| Column | 0 – 11
11-20 |
45 → 100
100 |
| Injection port | 150 | |
| Detector | 250 |
Detection: Flame ionisation.
Injection: 1 μL.
Retention time: Impurity C = about 7.5 min.
System suitability:
— resolution: minimum 5.0 between the peak due to impurity A (2 peak) and the peak due to impurity B (3 peak) in the chromatogram obtained with reference solution (a);
— signal-to-noise ratio: minimum 5 for the peak due to impurity C in the chromatogram obtained with reference solution (b).
Limits:
— impurities A, B: for each impurity, not more than the difference between the areas of the corresponding peaks in the chromatogram obtained with reference solution (a) and the areas of the corresponding peaks in the chromatogram obtained with the test solution (0.05 per cent V/V),
— impurity C: not more than the difference between the area of the peak due to impurity C in the chromatogram
obtained with reference solution (b) and the area of the corresponding peak in the chromatogram obtained with the test solution (2 ppm V/V),
— any other impurity: for each impurity, not more than the difference between the area of the peak due to impurity A in the chromatogram obtained with reference solution (a) and the area of the corresponding peak in the chromatogram obtained with the test solution (0.05 per cent V/V).
Matter insoluble in water
Dilute 1.0 mL to 20 mL with water R. The solution is clear (2.2.1).
Residue on evaporation
Maximum 50 ppm.
Evaporate 20.0 g to dryness on a water-bath and dry at 100-105 °C. The residue weighs a maximum of 1 mg.
Water (2.5.12)
Maximum 3 g/L, determined on 10.0 mL.
STORAGE
Protected from light.
IMPURITIES
Specified impurities A, B, C.
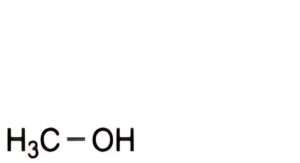
A. methanol,
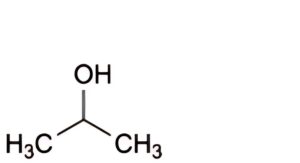
B. propan-2-ol (isopropanol),
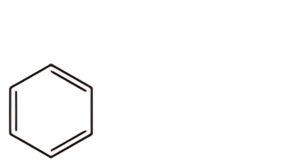
C. benzene.