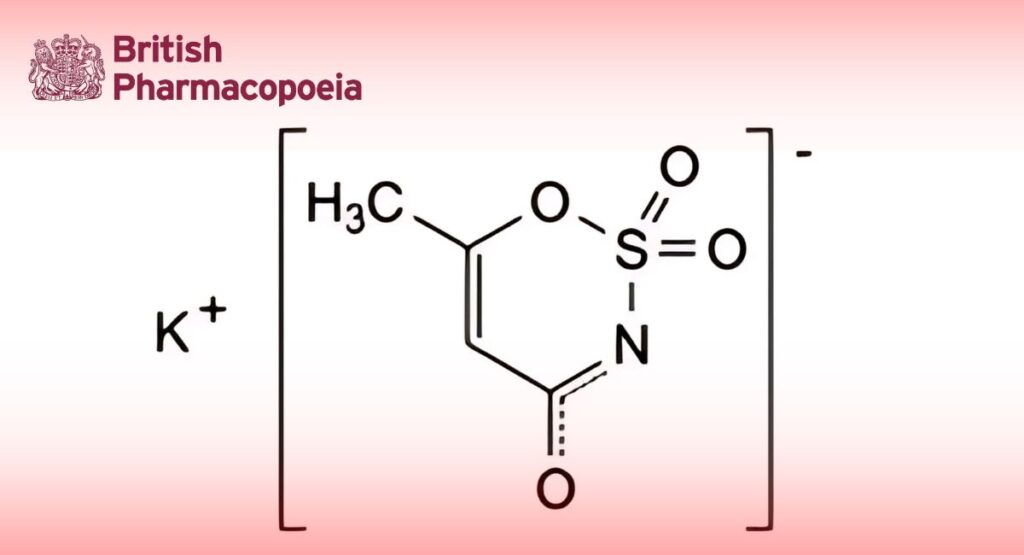
(Ph. Eur. monograph 1282)
C4H4KNO4S 201.2 55589-62-3
Action and use
Sweetening agent.
DEFINITION
Potassium 6-methyl-1,2,3-oxathiazin-4-olate 2,2-dioxide.
Content
99.0 per cent to 101.0 per cent (dried substance).
CHARACTERS
Appearance
White or almost white, crystalline powder or colourless crystals.
Solubility
Soluble in water, very slightly soluble in acetone and in ethanol (96 per cent).
IDENTIFICATION
First identification: A, C.
Second identification: B, C.
A. Infrared absorption spectrophotometry (2.2.24).
Acesulfame Potassium
Comparison acesulfame potassium CRS.
B. Thin-layer chromatography (2.2.27).
Test solution: Dissolve 5 mg of the substance to be examined in water R and dilute to 5 mL with the same solvent.
Reference solution (a): Dissolve 5 mg of acesulfame potassium CRS in water R and dilute to 5 mL with the same solvent.
Reference solution (b): Dissolve 5 mg of acesulfame potassium CRS and 5 mg of saccharin sodium R in water R and dilute to 5 mL with the same solvent.
Plate: cellulose for chromatography R as the coating substance.
Mobile phase: concentrated ammonia R, acetone R, ethyl acetate R (10:60:60 V/V/V).
Application: 5 μL as bands.
Development: Twice over 2/3 of the plate.
Drying: In a current of warm air.
Detection: Examine in ultraviolet light at 254 nm.
System suitability: Reference solution (b):
— the chromatogram shows 2 clearly separated zones.
Results: The principal zone in the chromatogram obtained with the test solution is similar in position and size to the principal zone in the chromatogram obtained with reference solution (a).
C. 0.5 mL of solution S (see Tests) gives reaction (b) of potassium (2.3.1).
TESTS
Solution S
Dissolve 10.0 g in carbon dioxide-free water R and dilute to 50 mL with the same solvent.
Appearance of solution
Solution S is clear (2.2.1) and colourless (2.2.2, Method II).
Acidity or alkalinity
To 20 mL of solution S add 0.1 mL of bromothymol blue solution R1. Not more than 0.2 mL of 0.01 M hydrochloric acid or 0.01 M sodium hydroxide is required to change the colour of the indicator.
Impurity A
Thin-layer chromatography (2.2.27).
Test solution: Dissolve 0.80 g of the substance to be examined in water R and dilute to 10 mL with the same solvent.
Reference solution (a): Dissolve 50 mg of acetylacetamide R (impurity A) in water R and dilute to 25 mL with the same solvent. To 5 mL of the solution add 45 mL of water R and dilute to 100 mL with methanol R.
Reference solution (b): To 10 mL of reference solution (a) add 1 mL of the test solution and dilute to 20 mL with methanol R.
Plate: TLC silica gel plate R.
Mobile phase: water R, ethanol (96 per cent) R, ethyl acetate R (2:15:74 V/V/V).
Application: 5 μL.
Development: Over 2/3 of the plate.
Drying: In air until the solvents are completely removed.
Detection: Spray with phosphoric vanillin solution R and heat at 120 °C for about 10 min; examine in daylight.
System suitability: The chromatogram obtained with reference solution (a) shows a clearly visible spot and the chromatogram obtained with reference solution (b) shows 2 clearly separated spots.
Limit:
— impurity A: any spot due to impurity A is not more intense than the spot in the chromatogram obtained with reference solution (a) (0.125 per cent).
Impurity B
Liquid chromatography (2.2.29).
Test solution: Dissolve 0.100 g of the substance to be examined in water R and dilute to 10.0 mL with the same solvent.
Reference solution (a): Dissolve 4.0 mg of acesulfame potassium impurity B CRS in water R and dilute to 100.0 mL with the same solvent. Dilute 1.0 mL of the solution to 200.0 mL with water R.
Reference solution (b): Dissolve 0.100 g of the substance to be examined in reference solution (a) and dilute to 10.0 mL with the same solution.
Column:
— size: l = 0.25 m, Ø = 4.6 mm;
— stationary phase: octadecylsilyl silica gel for chromatography R (3 μm).
Mobile phase: Mix 40 volumes of acetonitrile R and 60 volumes of a 3.3 g/L solution of tetrabutylammonium hydrogen sulfate R.
Flow rate: 1 mL/min.
Detection: Spectrophotometer at 234 nm.
Injection: 20 μL.
Run time: Twice the retention time of acesulfame.
Relative retention: With reference to acesulfame (retention time = about 5.3 min): impurity B = about 1.6.
System suitability:
— signal-to-noise ratio: minimum 10 for the peak due to impurity B in the chromatogram obtained with reference solution (a);
— peak-to-valley ratio: minimum 1.2, where Hp = height above the baseline of the peak due to impurity B and Hv = height above the baseline of the lowest point of the curve separating this peak from the peak due to acesulfame, in the chromatogram obtained with reference solution (b).
Limit:
— impurity B: not more than the area of the principal peak in the chromatogram obtained with reference solution (a) (20 ppm).
Fluorides
Maximum 3 ppm.
Potentiometry (2.2.36, Method I).
Test solution: Dissolve 3.000 g of the substance to be examined in distilled water R, add 15.0 mL of total-ionic-strength-
adjustment buffer R1 and dilute to 50.0 mL with distilled water R.
Reference solutions: To 0.5 mL, 1.0 mL, 1.5 mL and 3.0 mL of fluoride standard solution (10 ppm F) R add 15.0 mL of
total-ionic-strength-adjustment buffer R1 and dilute to 50.0 mL with distilled water R.
Indicator electrode Fluoride-selective.
Reference electrode: Silver-silver chloride.
Loss on drying (2.2.32)
Maximum 1.0 per cent, determined on 1.000 g by drying in an oven at 105 °C for 3 h.
ASSAY
Dissolve 0.150 g in 50 mL of anhydrous acetic acid R. Titrate with 0.1 M perchloric acid, determining the end-point potentiometrically (2.2.20).
1 mL of 0.1 M perchloric acid is equivalent to 20.12 mg of C4H4KNO4S.
IMPURITIES
Specified impurities A, B.
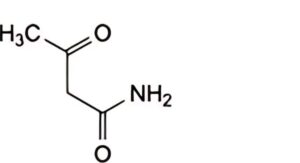
A. 3-oxobutanamide (acetylacetamide),

B. 5-chloro-6-methyl-1,2,3-oxathiazin-4(3H)-one 2,2-dioxide.