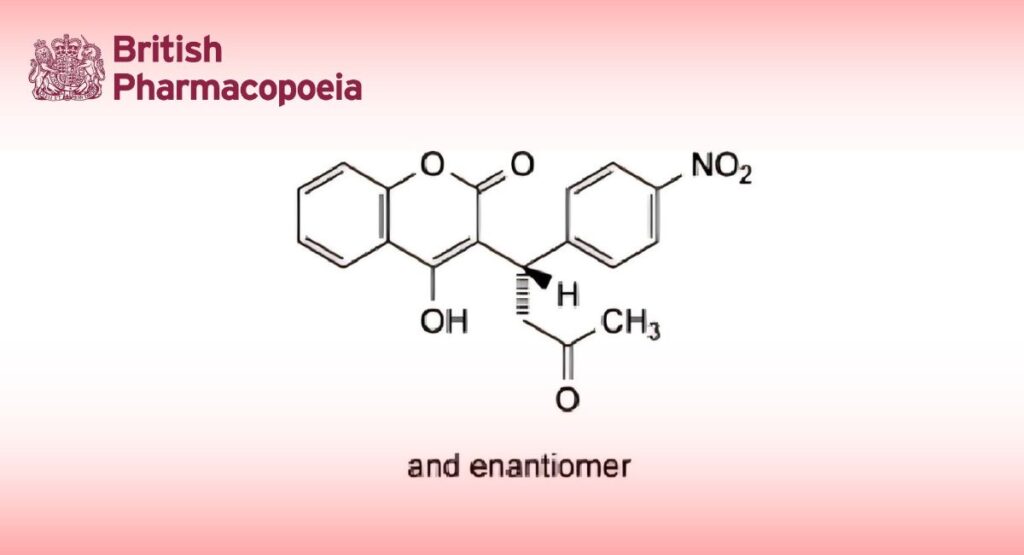
C19H15NO6 353.3 152-72-7
Action and use
Vitamin K epoxide reductase inhibitor; oral anticoagulant.
Preparation
Acenocoumarol Tablets
DEFINITION
Acenocoumarol is (RS)-4-hydroxy-3-(1-p-nitrophenyl-3-oxobutyl)coumarin. It contains not less than 98.5% and not more than 100.5% of C19H15NO6, calculated with reference to the dried substance.
CHARACTERISTICS
An almost white to buff powder.
Practically insoluble in water and in ether; slightly soluble in ethanol (96%). It dissolves in aqueous solutions of the alkalihydroxides. It exhibits polymorphism.
IDENTIFICATION
The infrared absorption spectrum, Appendix II A, is concordant with the reference spectrum of acenocoumarol (RS 001). If the spectra are not concordant, dissolve 0.1 g of the substance being examined in 10 mL of acetone and add water drop wise until the solution becomes turbid. Heat on a water bath until the solution is clear and allow to stand. Filter, wash the crystals with a mixture of equal volumes of acetone and water and dry at 100° at a pressure of 2 kPa for 30 minutes.
Prepare a new spectrum of the residue.
TESTS
Clarity and colour of solution
A. A 2.0% w/v solution in acetone is clear, Appendix IV A.
B. The absorbance of a 4-cm layer of a 2.0% w/v solution in acetone at 460 nm is not more than 0.12, Appendix II B.
C. A 2.0% w/v solution in 0.1M sodium hydroxide is clear, Appendix IV A, and yellow.
Light absorption
Absorbance of a 0.001% w/v solution in a mixture of 1 volume of 1M hydrochloric acid and 9 volumes of methanol at the maximum at 306 nm, 0.50 to 0.54, calculated with reference to the dried substance, Appendix II B.
Related substances
Carry out the method for thin-layer chromatography, Appendix III A, using the following solutions in acetone.
(1) 2.0% w/v of the substance being examined.
(2) 0.0020% w/v of the substance being examined.
CHROMATOGRAPHIC CONDITIONS
(a) Use as the coating silica gel GF254.
(b) Use the mobile phase as described below.
(c) Apply 20 μL of each solution.
(d) Develop the plate to 15 cm.
(e) After removal of the plate, allow it to dry in air and immediately examine under ultraviolet light (254 nm).
MOBILE PHASE
20 volumes of glacial acetic acid, 50 volumes of cyclohexane and 50 volumes of dichloromethane.
LIMITS
Any secondary spot in the chromatogram obtained with solution (1) is not more intense than the spot in the chromatogram obtained with solution (2) (0.1%).
Loss on drying
When dried to constant weight at 105°, loses not more than 0.5% of its weight. Use 1 g.
Sulfated ash
Not more than 0.1%, Appendix IX A.
ASSAY
Dissolve 0.6 g in 50 mL of acetone and titrate with 0.1M sodium hydroxide VS using bromothymol blue solution R3 as indicator. Repeat the operation without the substance being examined. The difference between the titrations represents the amount of sodium hydroxide required. Each mL of 0.1M sodium hydroxide VS is equivalent to 35.33 mg of C19H15NO6.